Research in the Wintrode laboratory
Serpins: folding, misfolding and conformational change
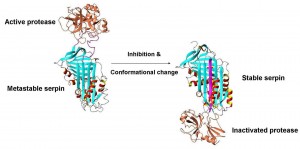
Serpins are a novel class of protease inhibitors that inactivate their target proteases through a unique mechanism. Unlike most proteins, inhibitory serpins fold to a metastable state rather than to their minimum free energy structure. Upon proteolytic cleavage of the flexible reactive center loop (RCL), serpins undergo a massive conformational change in which the N terminal portion of the RCL inserts into the central beta-sheet (sheet A) and becomea sixth strand, inactivating the covalently bound target protease in the process.
The serpinopathies are a group of inherited disorders that share as their molecular basis the misfolding and polymerization of serpins. Depending on the identity of the serpin, conditions arising from polymerization include emphysema, thrombosis, and dementia. The structure of serpin polymers is thus of considerable medical interest.