 The research approaches used in our laboratory include methodology of molecular and cellular biology, mouse models, bioanalysis, metabolic phenotyping, pharmacokinetics, pharmacodynamics, genetics, metabolomics, and clinical trials.
The research approaches used in our laboratory include methodology of molecular and cellular biology, mouse models, bioanalysis, metabolic phenotyping, pharmacokinetics, pharmacodynamics, genetics, metabolomics, and clinical trials.
Our laboratory has focused on the genetic determinants of drug-induced metabolic 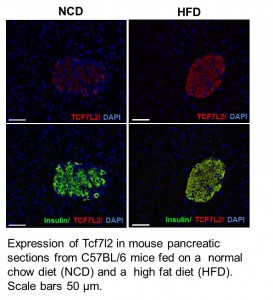 diseases and diabetes. For example, we have created a mouse model with genetic deficiency in Tcf7l2, a transcriptional factor protein involved in metabolic homeostasis and type 2 diabetes. By using this genetic model, along with other molecular, cellular, and clinical tools, we try to understand how the polymorphic TCF7L2 gene and its associated Wnt signaling pathway play a role in drug-induced metabolic disorders that are clinical prevalent but remain as an unaddressed critical issue. Our research effort in this direction also includes characterization of other genes’ function in drug-induced metabolic disorders, development of mouse models for metabolic research, and understanding epigenetic mechanisms of the metabolic phenotypes associated with drug use.
diseases and diabetes. For example, we have created a mouse model with genetic deficiency in Tcf7l2, a transcriptional factor protein involved in metabolic homeostasis and type 2 diabetes. By using this genetic model, along with other molecular, cellular, and clinical tools, we try to understand how the polymorphic TCF7L2 gene and its associated Wnt signaling pathway play a role in drug-induced metabolic disorders that are clinical prevalent but remain as an unaddressed critical issue. Our research effort in this direction also includes characterization of other genes’ function in drug-induced metabolic disorders, development of mouse models for metabolic research, and understanding epigenetic mechanisms of the metabolic phenotypes associated with drug use.
The second focus of our studies is on regulatory mechanisms of drug transporters. Despite their clinical significance, many drug transporters, particularly those uptake transporters including OCT1, are poorly characterized with respect to their regulation. The recently identified ischemia/reperfusion-inducible protein (IRIP) regulates a variety of transporters in vitro mediating membrane transport of 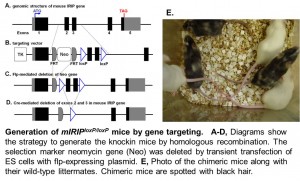 endogenous metabolites and xenobiotics. IRIP may represent a novel regulatory mechanism for transporter activity with a substantial impact on clinical drug therapy, and studies are highly warranted to characterize, as of yet unknown, the IRIP function in the body. We have created mouse models for this interesting gene and aim to characterize the first function of mammalian IRIP in the body. Additional effort includes investigation on how different factors such as genetic polymorphisms and diseases affect drug transporters, metabolizing enzymes and therefore drug responses.
endogenous metabolites and xenobiotics. IRIP may represent a novel regulatory mechanism for transporter activity with a substantial impact on clinical drug therapy, and studies are highly warranted to characterize, as of yet unknown, the IRIP function in the body. We have created mouse models for this interesting gene and aim to characterize the first function of mammalian IRIP in the body. Additional effort includes investigation on how different factors such as genetic polymorphisms and diseases affect drug transporters, metabolizing enzymes and therefore drug responses.
In the third focus, we are interested in drug-drug interaction that is mediated by a transport mechanism. We are particularly interested in the transporter 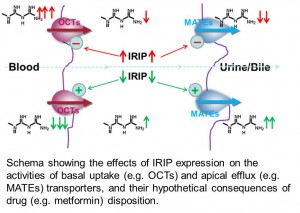 system consisting of Organic Cation Transporters (OCTs) and Multidrug and Toxin Extrusion proteins (MATEs) in the liver and kidney. A mouse model deficient of MATE function has been developed in our laboratory. With collaborators from FDA, we aim to understand the importance of OCTs and MATEs in clinical drug-drug interaction. Other effort includes investigation on pharmacological modulation on the transport of therapeutics into tumor tissues and on the role of drug transporters and regulatory molecules in anti-cancer drug resistance.
system consisting of Organic Cation Transporters (OCTs) and Multidrug and Toxin Extrusion proteins (MATEs) in the liver and kidney. A mouse model deficient of MATE function has been developed in our laboratory. With collaborators from FDA, we aim to understand the importance of OCTs and MATEs in clinical drug-drug interaction. Other effort includes investigation on pharmacological modulation on the transport of therapeutics into tumor tissues and on the role of drug transporters and regulatory molecules in anti-cancer drug resistance.
